Hepatocellular carcinoma is 10 times more likely to develop in the setting of nonalcoholic steatohepatitis than in simple fatty liver disease

A dangerous and growing problem
Overindulgence in the Western diet has led to an enormous increase in caloric consumption and obesity in the United States. Overall, 36.5% of Americans are obese, and obesity affects more than 40% of Americans aged 40 to 59 years.
Obesity is the primary driver of nonalcoholic fatty liver disease (NAFLD) and is estimated to impact a staggering 100 million Americans. Globally, the prevalence of NAFLD is more than 25%, making it the most prevalent liver disease worldwide.
In some patients NAFLD can progress to nonalcoholic steatohepatitis (NASH), which may in turn lead to a substantial number of cases of deadly hepatocellular carcinoma, or HCC, even in the absence of cirrhosis.
- Alarmingly, as many as 12% of adult Americans have NASH, putting them at increased risk for HCC
Current HCC surveillance guidelines do not account for NAFLD and NASH in the absence of cirrhosis
In addition to obesity, other metabolic comorbidities—particularly diabetes—occurring in the setting of NAFLD in the absence of cirrhosis can lead to HCC. However, these conditions are not factored into HCC surveillance guidelines, as patients who have NAFLD or NASH are not sufficiently represented in the clinical trials that support such guidelines. This clinical reality presents a challenge to surveillance programs for HCC that currently focus on patients who have cirrhosis.
The link between NAFLD and HCC took decades to establish
Foie gras, or fatty liver, was described by the French pathologist Pierre-Charles-Alexandre Louis in 1825. Thomas Addison introduced the term into the English language in 1836. The underlying clinical difference between the two forms of the disease—alcoholic fatty liver disease and NAFLD—is the patient’s heavy use of alcohol in alcoholic fatty liver disease. The histologic difference, however, was not characterized until liver biopsies had become common in the late 1950s, possibly delaying the clinical acceptance of NAFLD. In 2002, the risk of HCC developing in the setting of NAFLD was established by the results of two independent, seminal studies reported by Bugianesi et al and Marrero et al.
Metabolic risk factors for HCC and their population attributable fractions
The metabolic risk factors for HCC often occur in combination (cofactors) with dramatic effect. For example, new research has shown that the four horsemen of the “hepatocalypse”—diabetes, obesity, dyslipidemia, and hypertension—amplify the risk of HCC 2.6-fold among patients who have NAFLD when they are compared with patients who have NAFLD in the absence of such cofactors.
Population-attributable fraction of risk
Strong associations between metabolic risks and the development of HCC have been established in the last decade by examining the population-attributable fraction (PAF) of risk. Population-attributable fractions account for the strength of the association between exposure to an individual risk and outcome and the prevalence of the risk factor. Although the risk factors for HCC have been widely reported, it wasn’t until 2013 that PAFs were determined for HCC in the United States. In a study that year, nearly 7,000 patients who had a diagnosis of HCC were compared with more than 255,000 random control patients over a period from 1973 to 2007.
The study found that among individual risk factors for HCC, diabetes and obesity had the greatest PAF (36.6%), followed by chronic alcohol abuse (23.5%), hepatitis C virus (HCV) infection (22.4%), hepatitis B virus infection (6.3%), and genetic disorders (3.2%).
More recently, a study of PAFs for HCC in the United States confirmed these initial findings and found that the PAF for metabolic disorders, including diabetes and obesity, accounted for a remarkable 32% of the total HCC burden. Moreover, the metabolic disorder PAF increased from 26% to 36% over the course of the study. The runner-up PAF was chronic HCV infection, accounting for 20.5% of the HCC burden.
Overall HCC PAFs 2000 to 2011
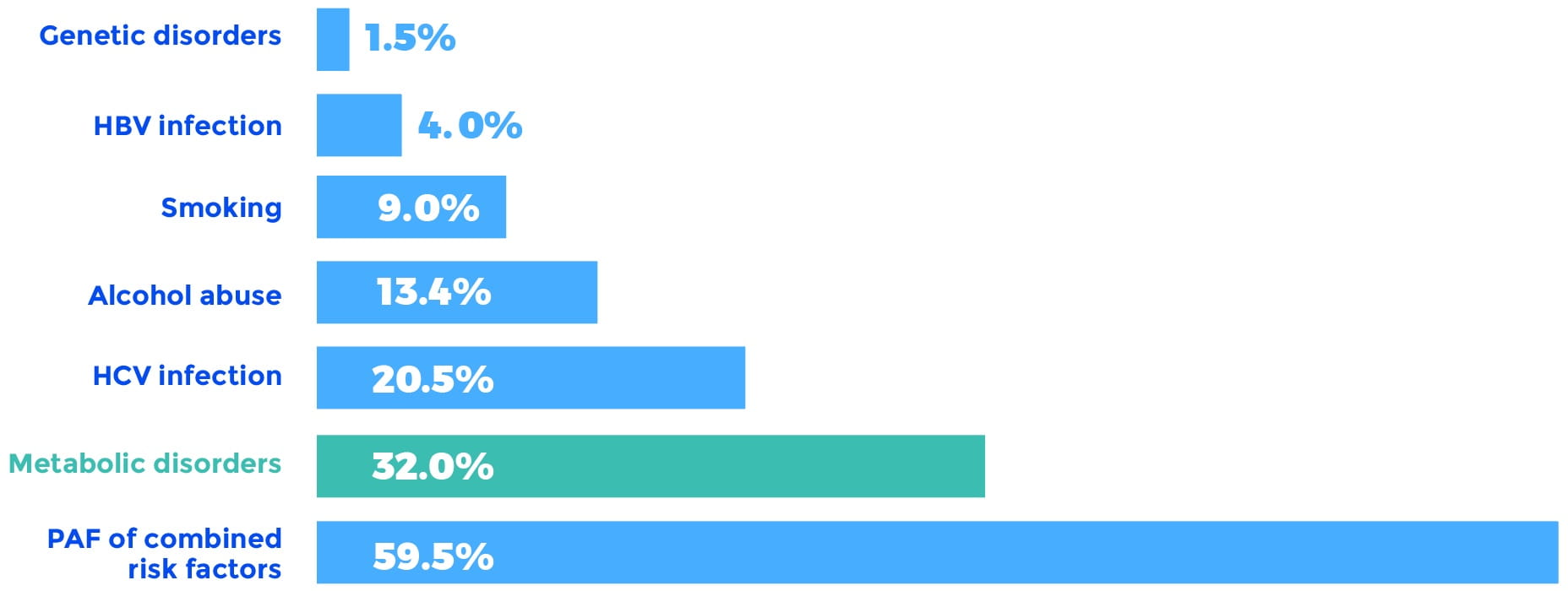
HBV, hepatitis B virus; HCV, hepatitis C virus; PAF, population-attributable fraction.
These data reveal that the highest PAFs for developing HCC are metabolic. With upwards of 70 million Americans living with NAFLD, many of whom are likely to have metabolic comorbidities, it should not be surprising that we now face a dangerous and growing problem.
In the context of these warnings and the enormous explosion of obesity, the lack of clinical attention paid to the metabolic risks for HCC and the absence of recommendations by scientific societies for the surveillance of HCC in the setting of NAFLD are striking.
Should surveillance for HCC be expanded to include more at-risk patients?
Several studies (reviewed by F. Negro and J. Debes) have confirmed that HCC can develop in patients who have fatty liver disease. These studies also confirm that HCC can develop in the absence of cirrhosis in patients who have fatty liver disease and associated metabolic traits.
Importantly, the study results revealed that the risk of HCC developing in the absence of cirrhosis was 5 times higher in patients who have HCC and NAFLD or metabolic syndrome than in patients who have HCV-related HCC.
Kanwal et al recently investigated the independent and combined effects of diabetes, hypertension, dyslipidemia, and obesity on the risk of cirrhosis or HCC developing among patients who had NAFLD. For both cirrhosis and HCC, disease progression occurred least in patients who had none or only one of the comorbidities; a stepwise progression of disease was found with each additional comorbidity. The results also establish that among patients who have NAFLD, diabetes had the strongest association with progression to HCC, whether cirrhosis was evident or not.
Currently, only patients who have cirrhosis are recommended for HCC surveillance, but that guideline may need to be expanded. All in all, the emerging studies underscore the need to include patients who have one or more metabolic comorbidities and NAFLD in the absence of cirrhosis in surveillance programs for HCC.
The implications of NAFLD and NASH on the surveillance of HCC
Because surveillance is not yet recommended for most patients who do not have cirrhosis, and the rate of adherence to current guideline-recommended surveillance is less than 30%, the challenge of adding in yet another population of at-risk patients may be daunting.
- A possible future approach to surveillance for HCC could be predicated on prioritizing patients who do not have cirrhosis but do have NAFLD and diabetes or a combination of several metabolic comorbidities. This approach to surveillance in the absence of cirrhosis is analogous to that suggested in international guidelines for patients infected with HBV who are at risk for HCC
Current surveillance methods are inadequate
Contributing to the surveillance challenge, the current primary surveillance tool (ultrasonography) performs poorly in patients who are obese or have NASH; therefore, an improved method of HCC surveillance that is simple to use and highly sensitive could benefit these and other patients at high risk for HCC.
“The ideal surveillance tool should be highly reproducible, not operator dependent (unlike abdominal ultrasonography), have good accuracy, and easy to implement in different clinical settings.” —Llovet, et al. Nature Reviews, 2021.
Visit us again soon at OncoguardLiver.com/education to learn more about the future of HCC surveillance.
The foregoing information is for informational purposes only and is not treatment advice for any patient. Physicians should use their clinical judgment and experience when deciding how to diagnose or treat patients.
Bibliography
Bugianesi E, Leone N, Vanni E, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134-140.
Debes JD, Boonstra A, de Knegt RJ. NAFLD-related hepatocellular carcinoma and the Four Horsemen of the Apocalypse. Hepatology. 2020;71(3):774-776.
EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma [published correction appears in J Hepatol. 2019 Apr;70(4):817]. J Hepatol. 2018;69:182-236.
Geh D, Anstee QM, Reeves HL. NAFLD-associated HCC: Progress and opportunities. J Hepatocell Carcinoma. 2021;8:223-239.
Geier A, Tiniakos D, Denk H, Trauner M. From the origin of NASH to the future of metabolic fatty liver disease. Gut. 2021;gutjnl-2020-323202.
Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380.
Kanwal F, Kramer JR, Li L, et al. Effect of metabolic traits on the risk of cirrhosis and hepatocellular cancer in nonalcoholic fatty liver disease. Hepatology. 2020;71(3):808-819.
Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6.
Loomba R, Lim JK, Patton H, El-Serag HB. AGA clinical practice update on screening and surveillance for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: expert review. Gastroenterology. 2020;158:1822-1830.
Makarova-Rusher OV, Altekruse SF, McNeel TS, et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer. 2016;122(11):1757-1765.
Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36(6):1349-1354.
McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73 (suppl):4-13.
Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14(1):124-31.e1.
National Institute of Diabetes and Digestive and Kidney Diseases website. Nonalcoholic fatty liver disease & NASH. Published November, 2016; available at https://www.niddk.nih.gov/health-information/liver-disease/nafld-nash/all-content. Accessed February 3, 2021.
Negro F. Natural history of NASH and HCC. Liver Int. 2020;40 Suppl 1:72-76.
Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS data brief, no 219. Hyattsville, MD: National Center for Health Statistics. 2015.
Rinella M, Charlton M. The globalization of nonalcoholic fatty liver disease: Prevalence and impact on world health. Hepatology. 2016;64:19-22.
Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263-2273.
Welzel TM, Graubard BI, Quraishi S, et al. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am J Gastroenterol. 2013;108(8):1314-1321.
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84.
