Evaluating an ALTernative to UltraSound: the ALTUS study for the surveillance of HCC

A novel solution may help improve routine HCC surveillance
Guidelines recommend routine, semiannual surveillance with ultrasonography with or without alpha fetoprotein testing for patients who are at risk for developing HCC, because regular testing enables detection of early-stage tumors, affording patients the opportunity to undergo potentially lifesaving treatment. However, the effectiveness of ultrasonography in detecting early-stage HCC may be limited by the performance of the technician and by each patient’s factors, such as obesity and nonalcoholic steatohepatitis. Moreover, many patients face barriers that inhibit their adherence and obstruct access to surveillance, including scheduling challenges, costs, and transportation concerns.
Evaluating a high-performance alternative to ultrasonography
To address the limitations and challenges with the current surveillance approaches to HCC, a multitarget liquid biopsy—a simple, convenient blood test—has been developed and clinically validated and is now available as the Oncoguard® Liver test for the detection of HCC.
- The validation study, which was published in 2021 in Clinical Gastroenterology and Hepatology, determined that the Oncoguard® Liver test detected HCC with 88% overall sensitivity, 82% early-stage sensitivity, and 87% specificity

The Oncoguard® Liver test is a part of a comprehensive approach to surveillance that includes a tailored patient engagement program that features easy-to-understand educational content and periodic reminders to help increase surveillance adherence.
The performance of the Oncoguard® Liver test for the detection of HCC is being evaluated further in a large HCC surveillance population.
ALTUS—an alternative to ultrasound imaging for the detection of HCC
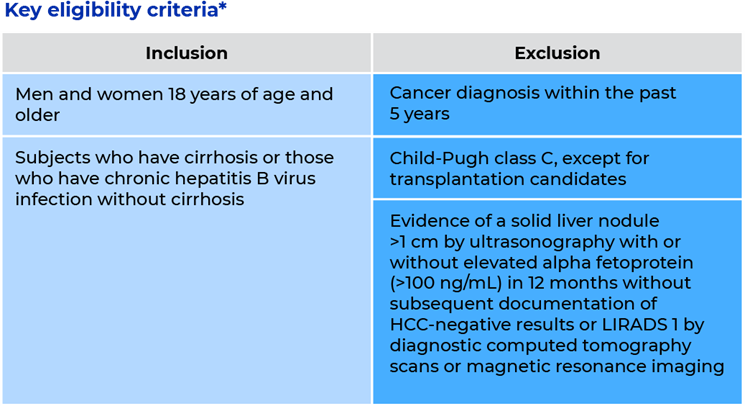
The ALTernative to UltraSound (ALTUS) study is a longitudinal, prospective, multicenter clinical trial (NCT05064553) designed to provide real-world performance data from the Oncoguard® Liver test. One of the study’s primary objectives is to assess the overall sensitivity and specificity of the Oncoguard® Liver test in detecting HCC in a surveillance population. Study description. The study is recruiting participants 18 years of age or older who are at risk for HCC and for whom HCC surveillance is appropriate, including participants who have cirrhosis and those who have chronic hepatitis B infection without cirrhosis. Approximately 3000 participants are expected to be enrolled, comprising approximately 2222 participants undergoing surveillance with ultrasonography and 778 participants undergoing surveillance with computed tomography scans or magnetic resonance imaging. A blood sample will be collected from all participants for performance evaluation in the Oncoguard® Liver test.
Now enrolling!
ALTUS is currently seeking to enroll eligible patients.
If you have patients who may qualify and are willing to consider participating, please contact Exact Sciences and reference the ALTUS study (NCT05064553).
*Additional eligibility criteria apply. Please see the study record registered at the National Library of Medicine (NCT05064553) for study details.
Visit us again soon at OncoguardLiver.com/education to learn more about the future of HCC surveillance.
The foregoing information is for informational purposes only and is not treatment advice for any patient. Physicians should use their clinical judgment and experience when deciding how to diagnose or treat patients.
The Oncoguard® Liver test is indicated as an aid in the detection of HCC for adults who have liver cirrhosis and/or chronic hepatitis B who are at risk for HCC.
Bibliography
American Cancer Society website. Liver cancer. Published April 1, 2019. Accessed February 24, 2022. https://www.cancer.org/cancer/liver-cancer
Chalasani NP, Porter K, Bhattacharya A, et al. Validation of a novel multitarget blood test shows high sensitivity to detect early stage hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2022;20:173-182.e7.
Choi DT, Kum HC, Park S, et al. Hepatocellular carcinoma screening is associated with increased survival of patients with cirrhosis. Clin Gastroenterol Hepatol. 2019;17:976-987.
Farvardin S, Patel J, Khambaty M, et al. Patient-reported barriers are associated with lower hepatocellular carcinoma surveillance rates in patients with cirrhosis. Hepatology. 2017;65:875-884.
John B, Porter K, Dahman B, et al. A prospective trial to evaluate the performance of the multitarget hepatocellular carcinoma blood test (mt-HBT) for screening at-risk patients: the ALTUS study. Abstract TPS486 presented at the American Society of Clinical Oncology Gastrointestinal Cancer Symposium, January 20, 2022. Accessed February 14, 2022. https://meetings.asco.org/abstracts-presentations/search?query=*&q+=TPS486&filters=%7B%22meetingTypeName%22:%5B%7B%22key%22%22Gastrointestinal%20Cancers%2Symposium%22 %7D%5D,%22meetingYear%22::%5B%7B%22key%22:2022%7D%5D,%22mediaType%22:%5B%7B%22key%22:%:22Abstracts%22%7D%5D%7D
Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6.
Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750.
Rahib L, Wehner MR, Matrisian LM, Nead KT. Estimated projection of US cancer incidence and death to 2040. JAMA Netw Open. 2021;4:e214708.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33.
