The COVID-19 pandemic reveals an opportunity to optimize the surveillance of HCC

Delays, deferrals, and opportunities
The imposition of physical distancing and stay-at-home orders intended to prevent the spread of COVID-19 have likely had unintended consequences for tens of thousands of at-risk patients who require liver care and surveillance to detect early-stage HCC. Two recent studies examined the impact of the pandemic on large numbers of patients who have liver disease and are at risk for HCC.
COVID-19 restrictions dramatically limit access to liver care and HCC surveillance
Toyoda and colleagues examined the changes in liver care before and after access to healthcare was restricted by measures intended to halt the COVID-19 pandemic. The researchers obtained data from three large medical centers in Palo Alto, CA, USA; Ogaki, Japan; and Singapore and focused on trends in the numbers of clinic visits and exams commonly performed for the assessment of HCC risk in patients who have liver disease:
-
Liver clinical visits for chronic hepatitis B and C, cirrhosis, and HCC
-
Abdominal ultrasound imaging for the surveillance of HCC
-
Computed tomography (CT) scans and magnetic resonance imaging (MRI)studies for surveillance and diagnosis of HCC
A trend analysis for overall clinic visits occurring during February, March, and April of 2018, 2019 (pre-pandemic), and 2020 (pandemic) comprised a total of 14,403 visits to the three sites.
The number of liver clinic visits for cirrhosis and HCC decreased 39% overall at the three sites and by 46.6% for the United States site (655 visits to 355 visits). In addition, the study found a “significant and notable” decrease in the number of clinic visits by patients aged 65 years or older in the United States; the age distribution across clinic visits in Japan and Singapore was stable during the same period.
Decline in overall liver clinic visits.

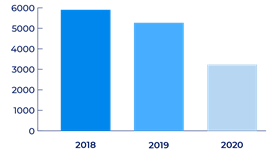
The number of abdominal ultrasound imaging visits (HCC surveillance) decreased overall (P trend = 0.004) and at the United States site (P trend = 0.010). The number of CT and MRI visits (diagnostic) also decreased overall and at the United States site (P trend = 0.007).
These analyses suggest that measures taken to limit the spread of the COVID-19 pandemic resulted in unintended consequences for patients who had liver disease without COVID-19. Significant decreases among all three indices indicate that pandemic restrictions limited access to liver disease care, HCC surveillance, and diagnostic procedures. The drop in surveillance is particularly distressing, given the lethality of HCC.
As the pandemic wanes and COVID-19 restrictions are eased, healthcare workers and office staff can assure patients that appropriate precautions remain in place for everyone’s safety. Importantly, patients who have liver disease should be reminded and encouraged to keep their appointments, including those for the surveillance of HCC.
Opportunity emerges to help optimize the surveillance and diagnosis of HCC
The decreasing trends in liver care presented above are supported by the results of a new national healthcare system study. Kim and colleagues evaluated the impact of the COVID-19 pandemic on the surveillance and diagnosis of HCC in pre-COVID-19 (n=94,612) and post-COVID-19 (n=88,073) Veterans Health Administration patients who had cirrhosis.
The authors reported that the HCC surveillance rate declined 44% (from 39% before the pandemic to 22%) during the pandemic study period (4/1/2020-9/30/2020). The decline in surveillance occurred irrespective of liver disease severity. The rate of new HCC diagnosis declined 13% (1.5% to 1.3%).
The study team noted that the recommendations for surveillance during the pandemic prioritize patients who are at the highest risk of HCC. Even with these recommendations, the subjects in the study at highest risk were not substantially more likely to undergo surveillance for HCC than those at lower risk. This finding uncovered an opportunity to apply the full measure of current surveillance recommendations. Accordingly, the authors identified subjects who were deemed to be at the highest risk for having HCC (FIB-4 score of at least 3.25 and an annual HCC risk of at least 1.5%) and suggested that patients who have these “predictors” could be further prioritized to undergo surveillance for HCC and help reduce delays in the diagnosis of HCC.
HCC surveillance should be simpler and easy to access
Protective measures against COVID-19 were instituted to reduce the spread of the novel coronavirus and consequently to “flatten the curve” of hospital admissions, allowing healthcare services to respond to the global crisis. The protective measures, however, appear to have had unintended consequences for many patients, and patients at risk for HCC are no exception.
Notably, the surveillance of HCC was often delayed and deferred as access to ultrasonography, CT scans, and MRI studies depend on the availability of technicians and equipment, both of which were prioritized to meet the medical needs of patients who had COVID-19 and required hospitalization.
The pandemic experience suggests that a simpler, easy-to-access approach to HCC surveillance could benefit at-risk patients during the next crisis and in routine practice, too.
Visit us again soon at OncoguardLiver.com/education to learn more about the future of HCC surveillance.
The foregoing information is for informational purposes only and is not treatment advice for any patient. Physicians should use their clinical judgment and experience when deciding how to diagnose or treat patients.
The Oncoguard® Liver test is indicated as an aid in the detection of HCC for adults with liver cirrhosis and/or chronic hepatitis B (HBV) who are at risk for HCC.
Bibliography
Gato S, Lucena-Valera A, Muñoz-Hernández R, et al. Impact of COVID-19 on liver disease: From the experimental to the clinic perspective. World J Virol. 2021;10:301-311.
Kim NJ, Rozenberg-Ben-Dror K, Jacob DA, et al. The COVID-19 pandemic highlights opportunities to improve hepatocellular carcinoma screening and diagnosis in a national health system: COVID-19 and HCC screening and diagnosis. Am J Gastroenterol. 2021;10.14309/ajg.0000000000001615.
Mani I, Alexopoulou A. Recent challenges facing patients with preexisting chronic liver disease in the era of the COVID-19 pandemic. Ann Gastroenterol. 2021;34:625-633.
Martinez MA, Franco S. Impact of COVID-19 in Liver Disease Progression. Hepatol Commun. 2021;5:1138-1150.
Mikolasevic I, Bozic D, Pavić T, et al. Liver disease in the era of COVID-19: Is the worst yet to come? World J Gastroenterol. 2021;27:6039-6052.
Toyoda H, Huang DQ, Le MH, Nguyen MH. Liver Care and Surveillance: The Global Impact of the COVID-19 Pandemic. Hepatol Commun. 2020;4:1751-1757.
World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19. Published March 11, 2020. Geneva, Switzerland. Available at https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Accessed February 7, 2022.
