From inception to practice—new insights from the development of a high-performance liquid biopsy test for the detection of early-stage HCC

Semiannual ultrasonography of the liver, with or without estimations of serum α-fetoprotein (AFP), is the standard of care approach to the surveillance of hepatocellular carcinoma (HCC) in at-risk patients. This approach, however, is limited in terms of patient access and the ability of ultrasonography to detect early- or curable-stage HCC.
Driven by a commitment to scientific rigor and innovation to improve the surveillance for HCC
The Oncoguard® Liver test demonstrates consistent results at every step—from development through validation
Recognizing the need to improve the available surveillance methods for HCC and the potential of DNA-based liquid biopsy testing, Exact Sciences embarked on a robust clinical development journey to develop and commercialize a high-performance liquid biopsy test for early-stage detection of HCC.
The program included efforts to identify and select methylated DNA and protein markers associated with HCC, algorithm development, and lab clinical validation of the test, which is now available as the Oncoguard® Liver test.
Rigorously designed sample collection study supported the validation of test performance in patients who have chronic liver disease
The performance evaluation of potential HCC markers was conducted in a multicenter study for the purpose of large-scale data collection (NCT03628651). The study enrolled a combined 700 cases and 1400 controls across sites in the United States, Europe, and Asia and represented one of the largest case-controlled studies of HCC in recent history. Specimens collected as part of this effort were then used across multiple phases of test development, including marker selection, algorithm development, and clinical validation studies.
Performance results from Oncoguard® Liver test were consistently high across all phases of development (Table 1).
Table 1. Results of the Oncoguard® Liver test across phases of development
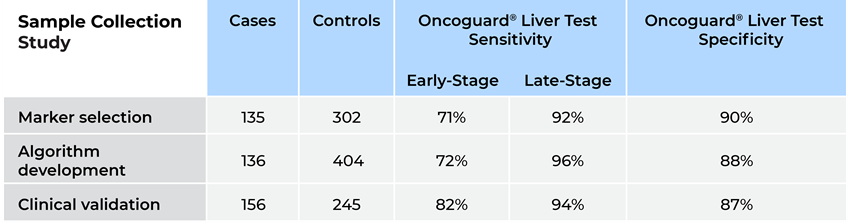
The lab clinical validation study results showed 88% overall sensitivity, 82% early-stage sensitivity, and 87% specificity.
Analysis of important patient subgroups provides new insights into early-stage sensitivity
Continuing the rigorous clinical evaluation of the blood-based tests for use in HCC surveillance, researchers conducted an additional performance analysis on the algorithm and validation cohorts of the early-stage sensitivity and specificity of the Oncoguard® Liver test, the GALAD score, and AFP performance stratified by patient and tumor characteristics.
The analysis focused on patient subgroups that are representative of at-risk populations. Performance was stratified by sex, age, race/ethnicity, body mass index, hepatitis B and C virus infection, evidence of alcoholic steatohepatitis, nonalcoholic fatty liver disease, and Child-Pugh classifications A and B. Sensitivity was further evaluated according to tumor size.
The analyses confirmed that the Oncoguard® Liver test had consistently high sensitivity for the detection of early-stage HCC across all patient subgroups regardless of disease etiology (Table 2 and Figure).
Table 2. Early-stage HCC sensitivity and specificity in at-risk populations
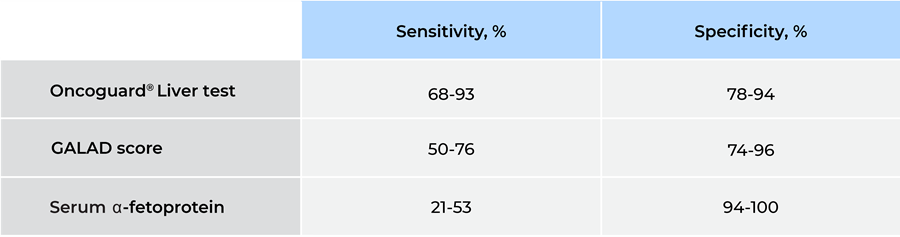
GALAD, gender, age, α-fetoprotein (AFP)-L3, and des-γ-carboxy prothrombin (DCP) model.
Early-stage HCC sensitivity for the Oncoguard® Liver test, GALAD score, and α-fetoprotein using established cutoff values across predefined patient subgroups.
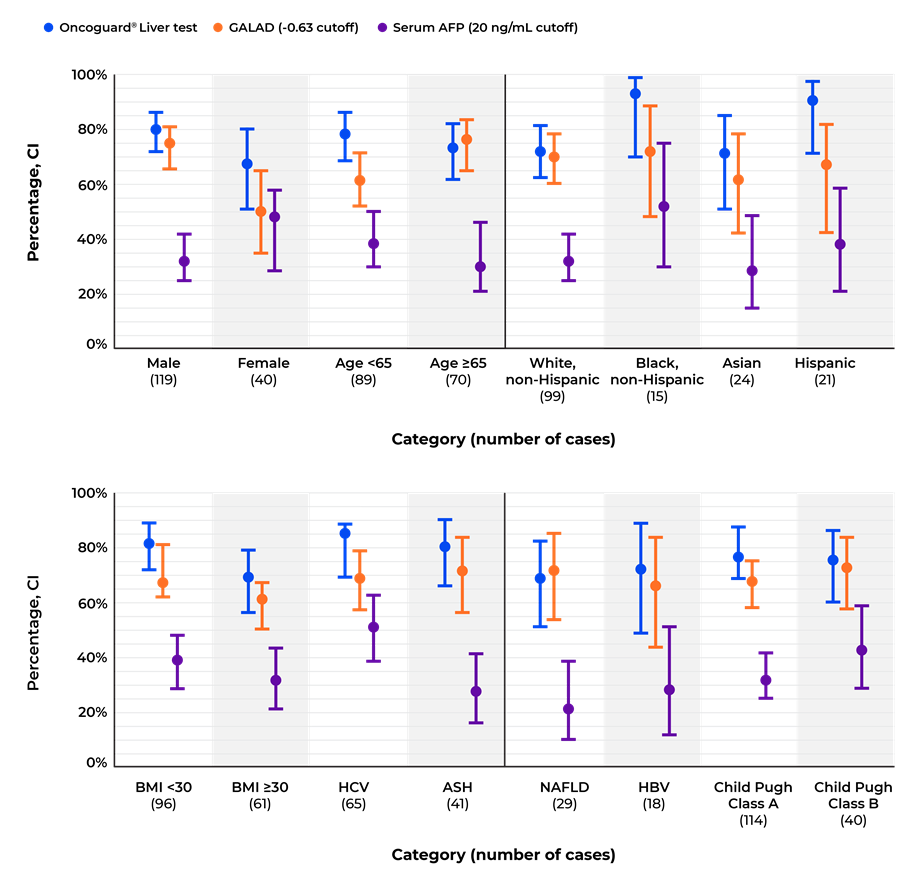
HCC, hepatocellular carcinoma; GALAD, gender, age, α-fetoprotein (AFP)-L3, and des-γ-carboxy prothrombin (DCP) model; CI, confidence interval; BMI, body mass index; HCV, hepatitis C virus; ASH, alcoholic steatohepatitis; NAFLD, nonalcoholic fatty liver disease; HBV, hepatitis B virus.
In addition, sensitivity increased with increasing tumor size for each method of HCC detection; however, point estimates were highest for the Oncoguard® Liver test in each size category (Table 3).
Table 3. Sensitivity by index tumor size
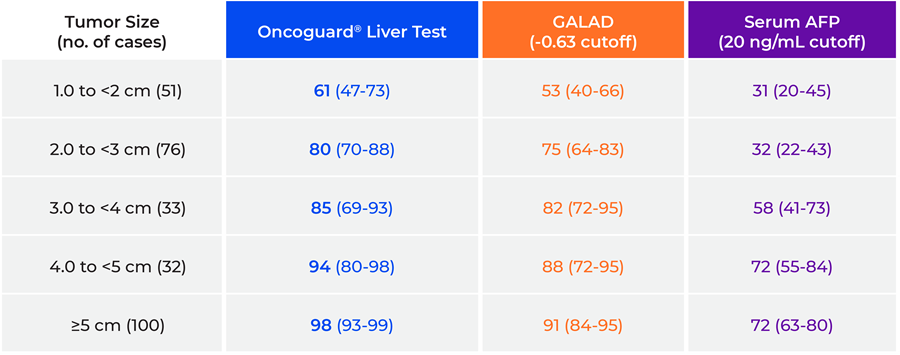
GALAD, gender, age, α-fetoprotein (AFP)-L3, and des-γ-carboxy prothrombin (DCP) model.
All values expressed as % (confidence interval) unless otherwise noted.
Exact Sciences is committed to robust scientific discovery and the continued generation of clinical evidence
A clinical study designed to further validate the performance of the Oncoguard® Liver test is underway. This study is expected to generate data that will provide additional insights beyond those gleaned from earlier studies. For example, new insights into the clinical performance of the Oncoguard® Liver test and its role in the surveillance of HCC are expected to emerge from the ongoing ALTernative to UltraSound (ALTUS) study.
The ALTUS study is prospectively investigating the overall performance of the Oncoguard® Liver test in a large patient population to help confirm the test’s performance characteristics, including the following:
- Overall sensitivity among patients who have HCC
- Specificity among patients who do not have HCC
- Early-stage sensitivity in a large HCC surveillance population
Today, patients at risk for HCC may benefit from a simple, highly sensitive alternative that helps address the limitations of ultrasonography in the detection of HCC.
Visit us again soon at OncoguardLiver.com/education to learn more about the future of HCC surveillance.
The foregoing information is for informational purposes only and is not treatment advice for any patient. Physicians should use their clinical judgment and experience when deciding how to diagnose or treat patients.
The Oncoguard® Liver test is designed as an aid in the detection of HCC for adults who have liver cirrhosis and/or chronic hepatitis B who are at risk for HCC.
Bibliography
Chalasani NP, Porter K, Bhattacharya A, et al. Validation of a novel multitarget blood test shows high sensitivity to detect early stage hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2022;20:173-182.e7.
Chalasani NP, Porter K, Book AJ, et al. The multi-target hepatocellular carcinoma blood test provides high sensitivity for detecting early-stage hepatocellular carcinoma across important patient subgroups. Presented at Digestive Disease Week, May 21 to 24, 2022. The abstract will be published in online supplements to Gastroenterology and GIE: Gastrointestinal Endoscopy.
Chalasani NP, Ramasubramanian TS, Bhattacharya A, et al. A novel blood-based panel of methylated DNA and protein markers for detection of early-stage hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2021;19:2597-2605.e4.
John B, Porter K, Dahman B, et al. A prospective trial to evaluate the performance of the multitarget hepatocellular carcinoma blood test (mt-HBT) for screening at-risk patients: the ALTUS study. Abstract TPS486 presented at the American Society of Clinical Oncology Gastrointestinal Cancer Symposium, January 20, 2022. Accessed February 14, 2022. https://meetings.asco.org.
Kisiel JB, Dukek BA, V S R Kanipakam R, et al. Hepatocellular carcinoma detection by plasma methylated DNA: Discovery, phase I pilot, and phase II clinical validation. Hepatology. 2019;69:1180-1192.
